Details of the Drug
General Information of Drug (ID: DMI347A)
| Drug Name |
Lamivudine
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
lamivudine; 134678-17-4; Epivir; Zeffix; Heptovir; Epivir-HBV; Hepitec; Heptodin; BCH-189; 3TC; Heptivir; CIS-LAMIVUDINE; (-)-2'-Deoxy-3'-thiacytidine; 4-amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one; GR-109714X; 3'-Thia-2',3'-dideoxycytidine; (-)-BCH-189; Lamivudine [USAN:BAN:INN]; GR109714X; beta-L-3'-Thia-2',3'-dideoxycytidine; beta-L-2',3'-Dideoxy-3'-thiacytidine; (-)NGPB-21; 136891-12-8; 2',3'-Dideoxy-3'-thiacytidine; (-)-BCH 189; UNII-2T8Q726O95; HSDB 7155; GR 109714X; DTHC; LMV; Lamivir; Zefix; BCH 189; BCH189; BCH-790; DRG-0126; Epivir (TN); Epivir(TM); GG-714; HHA & 3TC; HHA & Lamivudine; Heptovir (TN); Lamivudine & GNA; Zeffix (TN); Epivir-HBV (TN); Lamivudine [USAN:INN:BAN]; Lamivudine (JAN/USP/INN); Lamivudine, (2S-cis)-Isomer; Beta-L-2',3'-Dideoxy-3'-thiacytidine; Beta-L-3'-Thia-2',3'-dideoxycytidine; Beta-L-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-Saiko-To; (+/-)-(Cis)-1-[2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (+/-)-3TC; (+/-)-BCH-189; (+/-)-SddC; (-)-(2'R,5'S)-1-[2'-Hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine; (-)-1-((2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-NGPB-21; (-)-SddC; (-)-beta-L-2',3'-Dideoxy-3'-thiacytidine; (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; 2',3' Dideoxy 3' thiacytidine; 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-)-(Cis); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Galanthus Nivalis Agglutinin (GNA); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Hippeastrum hybrid agglutinin(HHA); 3TC & GNA; 3TC & SST; 3TC (AIDS INITIATIVE) (AIDS INITIATIVE); 3TC and NV-01; 3TC, Zeffix, Heptovir, Epivir, Epivir-HBV, Lamivudine; 4-Amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; Efavirenz/lamivudine/tenofovir fumarate
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency VirusHepatitis B virus
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
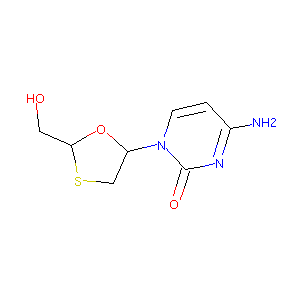 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 229.26 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lamivudine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
|---|---|---|---|---|---|
| 2 | Lamivudine FDA Label | ||||
| 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008 Aug;36(8):1616-23. | ||||
| 10 | The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007 Nov;64(5):645-54. | ||||
| 11 | Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm Drug Dispos. 2012 Apr;33(3):170-8. | ||||
| 12 | Model for intracellular Lamivudine metabolism in peripheral blood mononuclear cells ex vivo and in human immunodeficiency virus type 1-infected adolescents. Antimicrob Agents Chemother. 2006 Aug;50(8):2686-94. | ||||
| 13 | Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010 Jan;85(1):39-58. | ||||
| 14 | Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001 Dec;35(6):749-55. doi: 10.1016/s0168-8278(01)00218-5. | ||||
| 15 | Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286. | ||||
| 16 | Nucleoside reverse transcriptase inhibitors induce a mitophagy-associated endothelial cytotoxicity that is reversed by coenzyme Q10 cotreatment. Toxicol Sci. 2013 Aug;134(2):323-34. doi: 10.1093/toxsci/kft105. Epub 2013 May 2. | ||||
| 17 | Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005 Mar;20(2):139-46. doi: 10.1093/mutage/gei019. Epub 2005 Mar 22. | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 20 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 23 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 24 | Canadian Pharmacists Association. | ||||
| 25 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 26 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 27 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 28 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
